Carbonate dehydration diagenesis
I investigate carbonate chemistry and formation through field work, petrography, stable and clumped isotopes, various electron- and X-ray-based laboratory techniques, spectroscopy, and petrography
Understanding isotopic reordering during carbonate dehydration

Hexahydrated Ca-carbonate (ikaite) transforms to monohydrated Ca-carbonate (monohydrocalcite), which in turn transforms to calcite and aragonite pseudomorphs called thinolites (or glendonites). I am studying the isotope and phase changes resulting from this transformation in laboratory experiments utilizing oxygen and carbon stable isotopes, clumped isotopes, and X-ray diffraction. In addition, we study these transformation products in Mono Lake tufas, California, in order to understand hydrated carbonate formation and diagenetic dehydration conditions and textures.
Defining a new carbonate texture for paleoclimate reconstruction
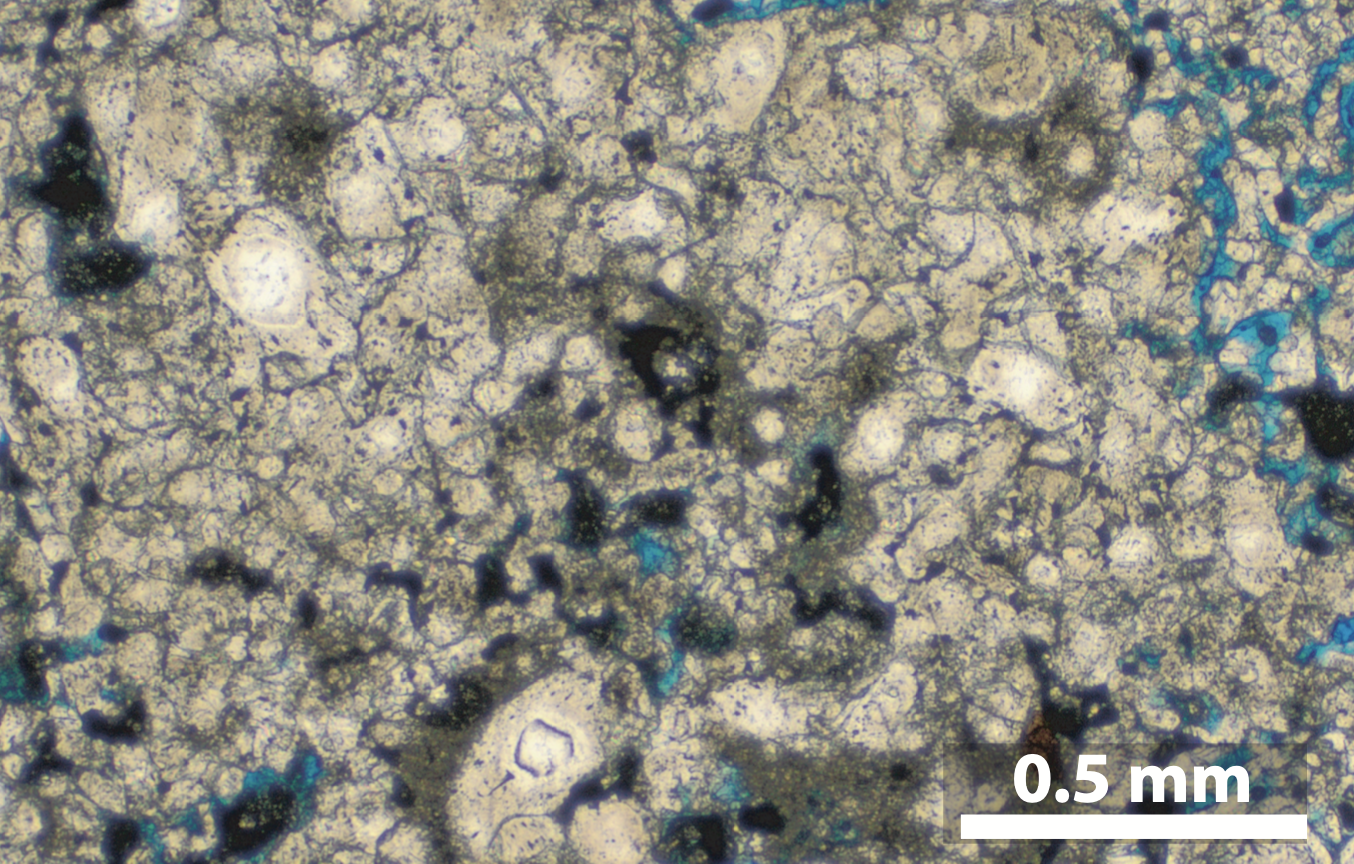
Hexahydrated Ca-carbonate (ikaite) transforms to monohydrated Ca-carbonate (monohydrocalcite), which in turn transforms to calcite and aragonite pseudomorphs called thinolites (or glendonites). We find that there is a characteristic microtexture in these pseudormophic crystals. It is recognized by its peculiar arrangement of rhombohedral and spheric calcite crystals. This microtexture can be used as evidence for carbonate dehydration at frigid paleoclimatic conditions.